You are here
R/V New Horizon Research Cruise - September 2009
 Date: Aug. 29 - Sept 11, 2009
Date: Aug. 29 - Sept 11, 2009
Chief Scientist: Tawnya Peterson
Research Vessel: R/V New Horizon
Document: R/V New Horizon cruise plan (.pdf)
Blog: Cruise blog
SCIENTIFIC OBJECTIVES 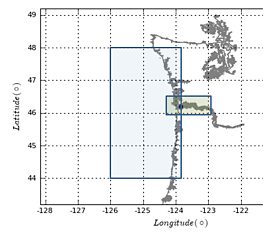 Figure 1. Map of area of operations. Blue box shows the coastal-offshore extent of our operations, while the green box indicates Columbia River and estuary sampling sites.
Figure 1. Map of area of operations. Blue box shows the coastal-offshore extent of our operations, while the green box indicates Columbia River and estuary sampling sites.
1.CTD casts and sensor-based measurements.
We will occupy a grid of stations over the Oregon and Washington continental shelf and slope (Fig. 1) to measure hydrographic (T, S, pressure), bio-optical (chlorophyll fluorescence, light transmission) and chemical (nitrate, phosphate, silicate, dissolved oxygen) parameters. An in situ nitrate sensor (ISUS) will be supplied by CMOP scientists and attached to the CTD rosette frame using stainless steel hose clamps. Based on operations in May 2009, the most feasible arrangement is for the ISUS itself to be attached to one side of the CTD-fluorometer package and the battery pack to be attached to the other side, below the Niskin bottles. We will combine in situ data with ADCP measurements of subsurface velocity to explore biophysical interactions
2. Water sampling.
We will collect water samples for DNA- and RNA-based microbial community analyses, measure uptake rates of carbon and nitrogen, and determine oxidation rates of methane by planktonic microbes. We will collect water samples for chemical analysis across environmental gradients in pelagic environments of the Columbia River, estuary, plume, and along established sampling lines on the Oregon and Washington coasts (Fig. 2). Some sampling will be coordinated with the R/V Point Sur to sample the Estuarine Turbidity Maxima (ETM) in the North and South Channels, and in the Columbia River Plume. a. Chemical and biological measurements. Microbiology samples (including DNA/RNA and chemistry) will be collected from three depths (surface [~2m], chl max [to be determined from the real-time profiles displayed on the lab computer during CTD casts], and bottom [bottom depth – 5 m]). Additional depths will be added to yield a more fine-scaled picture of vertical distributions of constituents of interest at select stations. These samples will be analyzed for a suite of chemical and biological concentrations (dissolved nutrients [nitrate, phosphate, ammonium, silicic acid], TDN/P, DOC, POC/N, SPM, Chl-a, HPLC pigments, flow cytometric cell counts, microscopic cell counts). Select samples will be analyzed for Colored Dissolved Organic Matter (CDOM) and dissolved CH4 (at sites along a transect from outside CRmouth, into the estuary and upriver to Beaver Army Terminal (BAT)). b. Rate measurements. Most samples will be analyzed for bacterial production rate (using 3H-leucine), and select samples will be analyzed for primary production rate (using NaH13CO3), nitrogen uptake rate (as Na15NO3, 15NH4Cl, and 15-N labeled urea), and for biogeochemical rate processes (CH4 oxidation [using 13CH4-labelled gas] and NH4 oxidation [using 15NH4Cl]). BP measurements will be conducted in an isotope van. Other measurements will be incubated in on-deck incubators. Grow-out experiments will be conducted to evaluate the rate of silicification in microplankton using on-deck incubators and to determine the metabolic response by phytoplankton to nutrient amendments from differing coastal environments. c. Phytoplankton assemblage structure, harmful algae, and phycotoxins. We will collect water samples from the Niskin bottles to evaluate phytoplankton assemblages in near-real time using a Flow Cytometer And Microscope (FlowCAM). Based on the results of the assemblages viewed by this instrument, we will collect samples for the analysis of domoic acid, yessotoxin, and other algal toxins potentially present in the waters of the Oregon/Washington coasts.
3. Surface flow-through system.
We will make continuous measurements of surface water chemistry with instrumentation attached to the continuous flow seawater system. These instruments will be ship-supplied fluorometer, flow meter, thermosalinograph, and oxygen sensor; and scientist-supplied nutrient analyzer (APNA) and flow-through cytometer (‘SeaFlow’).
4. Plume Feature Tracking.
Conduct Feature Tracking exercises using the SWAP system and model-based CORIE forecasts to identify sampling locations in the Columbia River Plume. Ship will cast CTD at pre-determined locations/times to test model.
5. SATURN station calibration.
Provide ground truth measurements for the SATURN biogeochemical observatory system with 24-hour CTD and water sample studies near the stations and in nearby locations to evaluate horizontal variability around moored sensor packages.
6. Estuary ETM and Myrionecta rubra sampling.
Sample the Estuarine Turbidity maxima in the north and south channels of the estuary with a modified CTD attached to pump tubing that is paid out during each cast (coordinate with R/V Point Sur). We will conduct vertical profiles to evaluate the distribution of the bloom-forming ciliate, Myrionecta rubra that often forms red streaks in waters of the Columbia River estuary.




