You are here
Week 1&2: Finding Nano-Fe
Before I start writing what I have been researching, here is some background info on reason why I am trying to "detect nanoscale Zero Valent Iron (nZVI)"
Background info:
nZVI has been known to be effective at breaking down Chlorine-based organic compounds (e.g. Trichloropropane). When nZVI is injected into the ground, it is highly favorable for us to know how fast and how far those nZVI are traveling horizontally from the injection site. To achieve that, I will be focusing on usage of Induced Polarization(IP) to detect nZVIs.
What I have been doing:
For first two weeks, I have been experimenting with phase differences and amplitude differences from IP. Currently the setup is as follows:
So I have been applying sinewave AC on outer two rods and observing the phase difference in the sine-wave and the amplitude reading at the two center rods. The liquid is usually 200mg Bicarbonate/L, and nZVI (Fe-BH) is changed to study the effect of chaging concentration of nZVI on phase difference and the amplitude.

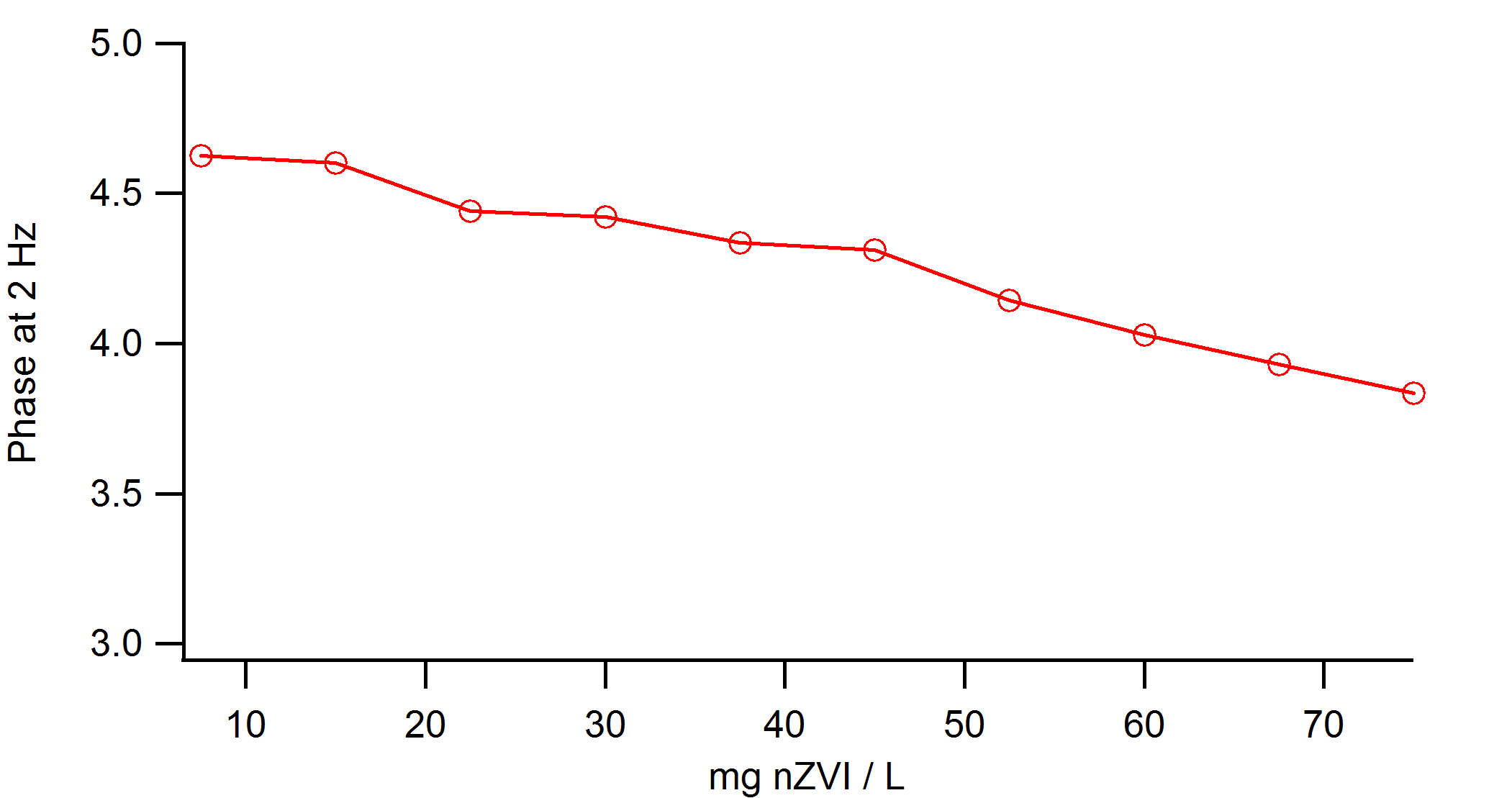
Above are two graphs that demonstrate the effect of conc. of nZVI.
1. In the top graph, the concentration is illustrated by the brightness of colors ([Lowest conc.] Red~Black [Highest conc]). x-axis has logarithmic frequency of applied AC. There seems to be a clear trend, which results in the next graph.
2. This graph is phase difference at Frequency=2Hz. It seems that with increasing concentration of nZVI, the phase difference decreases.
From now:
I will be attempting to collect more data to see if this pattern is observed in other environment (e.g. with NaCl ions present in the system, with different types of nZVI)



